HERNIA MESH LAWSUIT
Contact The Johnston Law Group for a free consultation about potential representation for a hernia mesh lawsuit.
Thousands of hernia mesh lawsuits have been filed against various manufacturers. Each year in the U.S., hernia mesh devices are implanted in more than 100,000 ventral hernia surgeries. The U.S. Food and Drug Administration (FDA) has made only a few recalls of the device, although there has been a large volume of complaints and hernia mesh lawsuits from patients who have experienced complications. The amount that a plaintiff may be awarded in a hernia mesh lawsuit differs based on a variety of factors. Among the lawsuits is one in which plaintiff Christopher Thorpe claimed that the implanted Kugel Mesh hernia patch broke inside of him, causing him to suffer a sepsis infection and severe internal injuries. Thorpe was awarded $1.5 million. Many hernia mesh lawsuits are being settled and many others are still being accepted for litigation.
A Pending Ethicon Trial
Among the numerous hernia mesh lawsuits is one filed by Matthew Huff, a patient who had hernia mesh implant surgery in 2013. He alleges that he has had multiple complications caused by the device. His claims include that, following the surgery, he went to the hospital with complaints of fever, nausea, severe pain, and chills. Doctors discovered the development of an infection around the Physiomesh hernia mesh device, which was manufactured by Ethicon.
The infection Huff suffered from resulted in the development of a fistula and several abscesses, which required additional surgery.
The original trial date was July 31, 2017, but it was reset. A 2018 trial date was postponed, with both the complainant and the defendant both asking for additional time, due to complexities of the case.
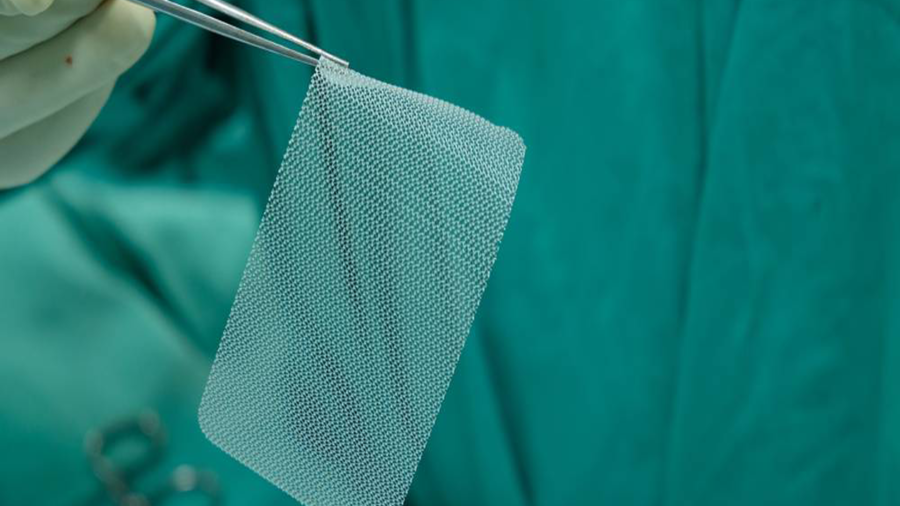
What is a Hernia Mesh Implant Device?
Hernia mesh or surgical mesh is a medical device that provides support to damaged or weakened tissue. The two types of materials used to construct most hernia mesh devices are synthetic materials and animal tissue.
Synthetic materials used for surgical mesh can be either non-knitted or knitted sheet forms. The materials can also be non-absorbable, absorbable, or a combination of the two.
Skin, intestine, and other types of animal tissue are used to make animal-derived absorbable mesh. The animal tissue is usually from a cow or pig and is processed and disinfected prior to use as a surgical implant device.
Non-absorbable mesh is considered a permanent implant because it indefinitely remains in the body. Absorbable mesh is not intended to provide long-term reinforcement. Over time, the mesh degrades and loses strength. It is intended that new tissue growth will provide strength to the repair site, as the absorbable mesh degrades.
What is a Hernia Mesh Implant Device?
According to a report in The Wall Street Journal, as many as 30% of all patients who undergo hernia mesh surgery end up experiencing chronic pain. Discomfort is the most common side effect of a defective hernia mesh device. Additional common issues follow:
- Mesh tearing or erosion
- Organic puncture or perforation
- Infection
- A physical or allergic reaction
- Chronic pain
- Intestinal fistulae, which is an abnormal opening in the digestive tract that causes gastric fluids to seep through the stomach or intestinal lining
- Adhesions of the mesh material to the bowel
- Lack of ingrowth of mesh
- Abscesses, which are painful collections of pus, usually caused by a bacterial infection
- Peritonitis or inflammation of the inner walls of the abdomen
- Hernia recurrence
Much more serious medical problems can occur. The following are among the most dangerous complications associated with hernia mesh surgery, though they are somewhat rare:
- Nerve damage
- Neurological changes
- Permanent or long-term liver or kidney problems
- Autoimmune disorders
- Sepsis, a potentially life-threatening condition in which a bacterial infection reaches the blood
FDA Information about Surgery Complications in Hernia Repair
According to the FDA, complications have been reported in connection with hernia mesh repair surgery. After conducting an analysis of adverse event reports on the medical device as well as scientific, peer-reviewed literature, the following are among the most common complications caused by surgical repair of hernias, with or without hernia mesh, says the FDA:
- Pain
- Infection
- Hernia recurrence
- Obstruction or blockage of the small or large intestine
- Adhesion in which scar-like tissue causes tissues to stick together
- Abnormal connection between intestines, vessels, or organs
- Perforation, which is a hole in neighboring organs or tissues
- Fluid build-up at the surgical site
Additional adverse events unique to surgeries with hernia mesh include mesh shrinkage or contraction and mesh migration.
The FDA recently reported that many complications with hernia repair that are reported are associated with products that have been recalled and are no longer on the market. An FDA analysis concluded that the main cause of obstruction complications and bowel perforation has been recalled mesh products.
Types of Hernias Mesh Implants are Used For
A hernia occurs when weakness in the stomach wall allows either tissue or part of the intestine to break through the weak area. Either stitching used to enclose the tissue or bowel or surgical mesh can be used to cover the hole and strengthen the area of weakness. Hernia mesh can be used for the following types of hernias:
- Inguinal hernia, located in the groin area
- Hiatal hernia, an abdominal hernia in the upper part of the stomach
- Abdominal hernia, located along the abdomen walls
- Incisional hernia, at the site of a previous surgery or injury
- Femoral hernia, which is more common among women, being located at the upper thigh around the outer groin or labia
- Umbilical hernia, which is usually near the naval or belly button and is therefore easy to see

Hernia Mesh Recalls, Injunctions, & Warnings
C.R. Bard/Davol was the first manufacturer with a recalled hernia mesh, and it was issued by the FDA in 2005. An extension to the recall was issued in the following year and also in 2007. Since that time, there have been other recalls, injunctions and warnings related to hernia mesh devices produced by various manufacturers.
Warnings
Several public warnings by the FDA about hernia mesh devices were issued in 2014. Companies whose products were the subject of the recalls include the following:
- Warnings about Bard products specified that there is a possible danger of breaking ring, which can result in various complications, including bowel perforation.
- The public was warned that Ethicon hernia mesh products have the possible danger of losing the coating of laminate.
- A warning about Atrium hernia mesh products involved improper packaging.
C-Qur Mesh Recall
The C-Qur Mesh device, which is manufactured by Atrium Medical Corporation, was recalled by the FDA in 2013. The safety of the product was not the issue, however. Instead, the recall was in regard to a packaging defect in which the device would get stuck to the inside lining, rendering it unusable. The FDA also received at least 35 complaints regarding human hair found in the C-Qur Mesh device.
But that was not to be the last of recalls from Atrium Medical Corporation. In 2015, following a multi-year investigation, a permanent injunction was issued by the FDA against Atrium. The investigation revealed that the manufacturer did not address multiple safety violations. As of 2017, Atrium Medical Corporation became one of several manufacturers facing defective hernia mesh class action lawsuits.
Physiomesh Recall
The Ethicon Physiomesh™ Composite Mesh was approved by the FDA on a fast-track 510(k) application, meaning that it was available to the public on the market with no additional safety studies because it was “similar” to other hernia mesh devices on the market.
It was voluntarily recalled by Johnson & Johnson in 2016. Two studies of the mesh were conducted and monitored, tracking the progress of patients with the mesh implants. Both studies revealed that patients using the product had a higher incidence of new surgery or hernia recurrence as compared with patients using other products.
Versatex Monofilament Mesh
After the manufacturer noticed that hernia recurrence was reported by a high number of patients, the FDA issued a recall notification for Versatex Monofilament Mesh in 2018.
Signs of Hernia Mesh Rejection
If you have had a hernia mesh implant, it is important to speak to your doctor without delay if you experience any of the following conditions because they are signs of hernia mesh rejection:
- A rash or redness around the surgery area
- Severe abdominal pain
- Nausea
- Unexplained cramps
- A fever
Surgical mesh is intended to reduce, not eliminate, the possibility of recurrence. Your hernia could return after surgical hernia mesh has been implanted. The chances of recurrence are further increased, if you have a defective hernia mesh device.
A bacterial infection is one of the most common causes of hernia mesh rejection. The first 30 days after your surgery is when you are at greatest risk for a bacterial infection, according to medical experts at John Hopkins Medicine.
You may have an autoimmune response to hernia mesh, in which your immune system attacks the medical device as though it were an infection. This causes swelling and inflammation. More surgery may be needed as a result of an autoimmune response.
The Value of Hernia Mesh Cases
In a product liability litigation like hernia mesh lawsuits, there are various types of damages available, including the following:
- Compensatory damages are awarded to repay the physically injured for the cost of medical bills, pay lost from taking too much sick time, and future loss of pay, due to a reduced capacity to work. The scope of compensatory damages can also include emotional distress for anxiety-related symptoms.
- Pain and suffering refer to physical pain the complainant experiences as a result of the injury. Types of physical pain include discomfort, pain, aches, scarring, and limitations when performing normal activities.
- Loss of consortium is when a spouse has been deprived of comfort, affection, and love in a family relationship as a result of the spouse’s injury.
- Punitive damages are awarded to a complaint for the purpose of punishing the defendant, if it has been determined that the defendant behaved in a wanton or reckless way that, therefore, intentionally caused harm.
When evaluating a case for consideration of a hernia mesh lawsuit, the following elements are considered:
- Which company manufactured the hernia mesh device can potentially improve the chances of success in a product liability lawsuit. For example, was the device voluntarily pulled from the market or recalled by the FDA? Are there important studies with adverse conclusions showing that the specific type or model of hernia meshed you used is associated with serious complications?
- How serious are the injuries suffered? Among the essential factors involved in assessing the value of a hernia mesh claim is the nature and severity of the plaintiff’s injuries. For example, if a plaintiff has endured prolonged hospital stays, has endured multiple surgeries, and has sustained permanent damage, financial awards for the plaintiff are most likely higher.
- How costly are the medical bills? The total amount paid for doctor visits, hospitalizations, medications, diagnostic testing, assistive medical devices, and surgeries comes into play, when evaluating a hernia mesh lawsuit.
- How high is the cost of lost and future wages? The total in lost income factors into the jury award or settlement of a product liability case. For example, how long has the plaintiff been unable to work? Can the plaintiff return to previous employment or has permanent disability prevented it? Plaintiffs are entitled to financial recover for past, present, and future loss of wages.
- To what extent does the injury negatively impact daily life and the ability to work? Compensation awards are higher, for example, if hernia mesh complications have resulted in lifelong digestive problems or chronic infections.
Product Liability
Product liability holds a manufacturer or seller responsible for placing a defective product on the market for consumer use. By law, products must meet the ordinary expectations of consumers. If a product is known to have an unforeseen danger or defect, it does not meet reasonable expectations and does not belong on the market.
Statute of Limitations
Product liability lawsuits, such as hernia mesh lawsuits, come under a “discoverability rule.” This means that once you learn that your symptoms stemmed from hernia mesh problems, you have a limited amount of time in which to file a hernia mesh product liability claim. The specific laws are determined by the state you live in. We recommend that you contact a product liability attorney as soon as possible, to ensure that you don’t make your claim too late.
Source
- https://www.giejournal.org/article/S0016-5107(07)02043-3/abstract
- https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/herniasurgicalmesh/default.htm
- Endo to Pay $830 Million to Settle Claims Over Devices. New York Times. April 30, 2014.
- Case Management Order No. 1. In re: Physiomesh Litigation (Flexible Composite Mesh). Case No. 627. Superior Court of New Jersey Law Division: Atlantic County.
- Long-term Recurrence and Complications Associated With Elective Incisional Hernia Repair. JAMA. 2016;316(15):1575-1582. doi:10.1001/jama.2016.15217
- J&J’s Ethicon recalls Physiomesh flexible composite hernia mesh. Mass Device. June 20, 2016.
- Hernia mesh litigation mounts. NH Business Review. February 1, 2018.
- J&J to Pay $120 Million to Settle Thousands of Vaginal Mesh Lawsuits. New Brunswick Today. February 4, 2016.
- Degradation of mesh coatings and intraperitoneal adhesion formation in an experimental model. British Journal of Surgery. 2009 Mar;96(3):305-13. doi: 10.1002/bjs.6446.
- District Court Enters Permanent Injunction Against New Hampshire Company and Senior Executives to Stop Distribution of Adulterated and Misbranded Products. February 3, 2015.
- In RE: Ethicon Physiomesh Flexible Composite Hernia Mesh Products Liability Litigation.
- Atrium Medical Corp. C-Qur Mesh Products Liability Litigation.
- Class 2 Device Recall CQUR Edge Mesh.U.S. Food & Drug Administration. July 2013.
- Hernia Surgical Mesh Implants.U.S. Food & Drug Administration. February 4, 2018.
- Johnson & Johnson Hit With $35 Million Surgical Mesh Implant Verdict. Mass Tort Nexus. March 29, 2018.
Written by:

Stephanie McHugh
Stephanie McHugh is a professional writer who specializes in legal articles, technical blogs, and website copy. She is a former Official Court Reporter for a District Court in Houston, Harris County, Texas; newspaper columnist; and teacher. Stephanie is a professional writer with extensive experience reporting on health-related topics for publication. She developed an interest in health-related litigation as a court reporter while taking depositions for a class action lawsuit. Through her writing, she has been glad to help raise awareness about public health threats, to benefit victims.

HERNIA MESH LAWSUIT
Contact The Johnston Law Group for a free consultation about potential representation for a hernia mesh lawsuit.