Written by Emre Ertugrul
Smith & Nephew’s first-generation knee replacement device was recalled on Oct. 1 as the Food and Drug Administration announced the Class 2 device recall online, following a series of research and tests regarding the high failure rate of the device.
Smith & Nephew’s JOURNEY Bi-Cruciate Stabilized (BCS) Knee system was the first-generation version of the JOURNEY II BCS which replaced the former on global market.
The Field Safety Notice (FSN) sent to risk managers on June 13, requested customers to inspect and locate the affected devices with the purpose of their returns to Smith & Nephew, the FDA stated in the report. The same notification was also sent to surgeons.
Earlier in June, Smith & Nephew issued a safety notice regarding the high rate of failure of its first-generation device, due to early femoral and tibial insert component complications.
Total knee replacement is the process in which part of the knee joint is replaced with artificial knee parts – a common process that is experienced by hundreds of thousands of Americans each year.
“Review of post-market surveillance data” resulted in the urgent safety notice as the device reportedly caused unexpectedly earlier needs in patients for painful revision surgeries. The manufacturer stated that JOURNEY BCS device had the same reasons for revision as other knee systems on the market, however, the first-generation device had “a rate higher than expected.”
The first-generation system was released for market globally in 2013 and is no longer for sale.
In August, the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK issued a warning for providers and physicians that Smith & Nephew’s first-generation device has a 50% greater risk to cause early failure.
A clinical follow-up process conducted by Smith & Nephew concluded that the JOURNEY BCS Knee system has a revision rate over 1.5 times the average revision rates in the National Joint Registry of the UK and Australian Orthopaedic Association National Joint Replacement Registry.
The Journey II BCS Knee system will not be affected by the action.
Written by

Emre Ertugrul
Emre Ertugrul is a reporter for Safetywatch.org, covering controversial drugs and medical devices, reporting on health policy and the FDA. He studied journalism with concentration in investigative reporting at Boston University. Previous experience with the New England Center for Investigative Reporting include tax issues, racial profiling and criminal justice. He also worked as an international news intern at Milliyet Newspaper and is currently one of the editors for Gazet.com.
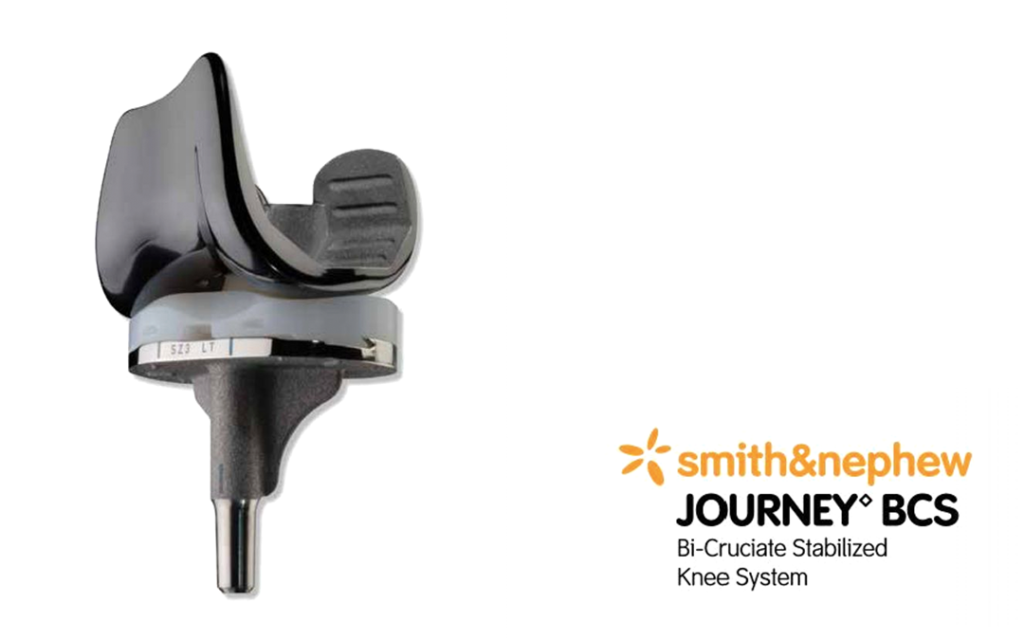
JOURNEY 1st gen BCS Knee System by Smith & Nephew Recall
Get a Free Case Evaluation Now!