The third bellwether MDL trial on C.R. Bard’s controversial anti-clotting filter device was ended with the company’s clearance on Oct. 5 in the District Court of Arizona, which is notorious for having very conservative jury panels. The lawsuit was filed over claims that manufacturer’s IVC filter device was defective in breaking and sending its pieces toward different parts of body.
Plaintiff Lisa Hyde suffered from the implanted retrievable device’s fractured legs that sent a metal shard into her heart. She had to undergo a surgery to remove the pieces in her ventricle in 2014. The jury did not find Bard liable for Hyde’s sufferance.
She had to live with this life-threatening condition because her doctors had no way to remove the metal shard from her heart. Fearing death every day, not knowing how long she could survive with this metal shard perforating in her heart, Hyde wrote daily letters to each of her small children for each of their anticipated life milestones, telling them to have her words of encouragement, to remember she loved each of them and to understand that she is always with them, even if only in spirit.
But she was in tears when the jurors decided that the manufacturer was not responsible and negligent.
Hyde, who is a married mother of three, was implanted with a retrievable Bard G2X IVC filter (See figure A) while she lived in Wisconsin. She subsequently moved to Nevada, and while there, a CT scan indicated that the Defendant’s IVC filter tilted then perforated the inferior vena cava (IVC) wall fracturing one of the filter’s struts sending a metal shard migrating to and lodging in Plaintiff Lisa Hyde’s right ventricle of her heart (See figure B).
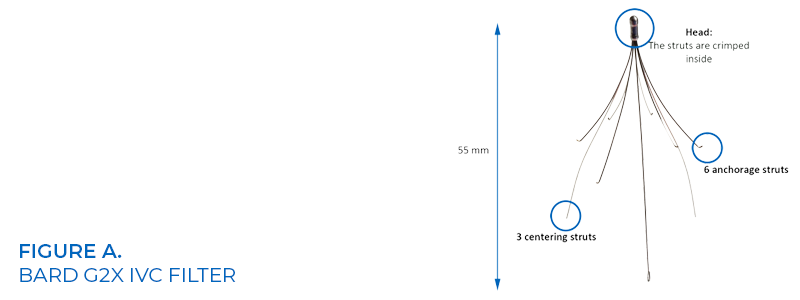
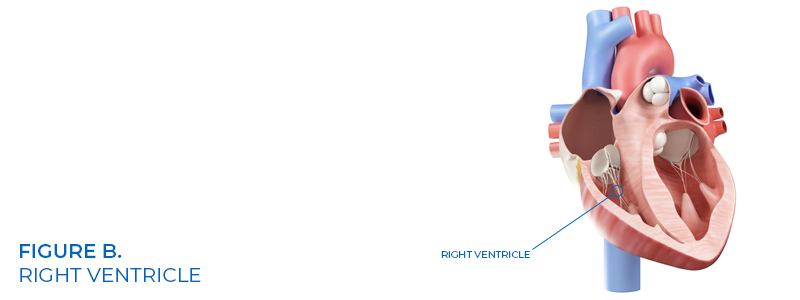
Attorneys for plaintiff Hyde argued defendant Bard’s IVC filters did not undergo safety or efficacy clinical testing and implanting defendant’s device was nothing short of human experimentation.
As many manufacturers did, Bard restated that the device had possible risks of breakage. Defendant’s attorneys stated that Bard IVC filters save lives because blood clots lead to death. However, Hyde’s lawyers’ argument was regarding the defectiveness of Bard’s retrievable device and the new component of the vena cava filter known as caudal anchors – which was stated to be necessary.
Bard engineers had previously requested to add the component to the feet of the filter; however, the company used the anchors only on the new Meridian series while keeping on the market the previous designs such as G2 – which did not have the necessary component.
Manufacturer Bard had no trouble in receiving the market approval for its device through the substantially equivalent method, known as the 510(k) process. The first method is through the Premarket Approval, or the PMA.
The PMA is the Food and Drug Administration process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices such as Bard’s G2X IVC filter. Class III devices are those hat support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.
Due to the level of risk associated with Class III devices, the FDA has determined that general and special controls alone are insufficient to assure the safety and effectiveness of Class III devices. Therefore, these devices require the PMA application under section 515 of the Food, Drug and Cosmetic Act in order to obtain marketing approval to place their products into the stream of commerce.
The 510(k) process in essence fast-tracks the commercial use approval process without requiring the company to perform clinical or safety testing, so long as the product is as safe and effective and is substantially equivalent to a PMA device that has already been on the market legally.
Defendant Bard introduced the first retrievable IVC filter even though the PMA IVC filter approval by the FDA was for a permanent, nonremovable filter. Bard represented to the FDA that their filter was permanent, safe, effective and substantially equivalent to the PMA device, resulting in an approval without any clinical testing.
After several subsequent 510(k) applications to market their filter as retrievable, Bard began marketing its IVC filter as retrievable with the FDA consent.
It took almost 6 years until the FDA issued Bard a warning in 2010, detailing the serious injuries and death risks associated with the manufacturer’s IVC filter fracture, migration and perforation as the company had received series of hazardous event reports.
The FDA updated the warning in 2014, adding the serious problems that strut fracture, perforation and migration can cause in the right atrial wall and the heart.
Despite earlier adverse events and Hyde’s lawyers adding that Bard should have withdrawn the earlier series until they developed a safe design, defendant Bard has not taken their IVC filters off the market, thus reaching an estimated annual of $3 billion.
The first bellwether trial of Bard IVC filters ended with a plaintiff’s verdict of $3.6 million compensation to Sherr-Una Booker. Booker required a heart surgery after a Bard G2 filter was implanted. A subsequent case was dismissed as being barred by the statute of limitations.
Two more bellwether trials are going to be held in February and May 2019.
Written By:

Emre Ertugrul
Emre Ertugrul is a reporter for Safetywatch.org, covering controversial drugs and medical devices, reporting on health policy and the FDA. He studied journalism with concentration in investigative reporting at Boston University. Previous experience with the New England Center for Investigative Reporting include tax issues, racial profiling and criminal justice. He also worked as an international news intern at Milliyet Newspaper and is currently one of the editors for Gazet.com.
GET A FREE IVC FILTER CASE REVIEW
If you or a loved one experienced a complication after getting an IVC filter, you may be eligible for a compensation.


