Thousands of IVC filter lawsuits have been filed against the manufacturers of a medical device designed to protect the lungs from blood clots. Severe IVC filter complications were reported to the United States Food and Drug Administration (FDA) beginning in 2005. Since that time, thousands of patients have reported severe problems resulting from being implanted with IVC filters. Approval by the FDA is still in effect and many of the devices are still being widely used, though at least 39 deaths are known to be associated with IVC filter implants, as of May 2018.
Patients report such problems as IVC filter fractures, migration of the device to other parts of the body, embolization, movement of the entire device or fragments of it into the lungs and heart, or perforation. IVC filter complications patients have experienced include organ damage, chronic pain, stroke, pulmonary embolism, severe internal bleeding, and death.
Plaintiffs claim through IVC filter lawyers, among other things, that the manufacturers of IVC filters were aware of the defectiveness of their products and failed to inform doctors and patients about the seriousness of the risks. Manufacturers of the device include: C.R. Bard, Cook Medical, Cordis (OptEase and TrapEase), and Boston Scientific (Greenfield). The majority of IVC filter lawsuits are pending against C.R. Bard and Cook Medical.
IVC Filter Settlements with C.R. Bard
IVC Filter Settlements with C.R. Bard
In 2011, the first IVC filter lawsuit was filed by Plaintiff Lisa Davis in Pennsylvania against C.R. Bard. Davis had a G2 series IVC filter implanted in 2006. She began to experience ongoing heart issues after the medical device fractured and migrated to her heart in 2008. In 2013, the judge in the Eastern District of Michigan Southern Division U.S. District Court case was notified that a settlement was reached between C.R. Bard and Davis, in an undisclosed amount.
In 2013, Kelly Vlasvich and her husband filed a lawsuit against C.R. Bard with the Northern District of Illinois U.S. District Court. A G2 filter that had been implanted fractured in 2011, which reportedly damaged Vlasvich’s lungs and heart. The case was closed in 2015 after the parties jointly filed a stipulation of dismissal.
In 2015, Plaintiff Kevin Philips brought a case against C.R. Bard before the U.S. District Court for the District of Nevada. The Recovery IVC filter perforated Philips’ heart. In just 10 days after the filing, C.R. Bard settled the case with Philips.
IVC Filter Class Action Lawsuits against C.R. Bard
Among the class-action IVC filter lawsuits are three pending against C.R. Bard. The class action suits are in California, Florida, and Pennsylvania. Plaintiffs claim that the company was negligent, concealing data about defects in their IVC filter devices from doctors and patients. The lawsuits argue that even in cases in which G2, G2 Express, and Recovery filters did not migrate or fracture, patients are entitled to medical monitoring.
More than 100 plaintiffs seeking more than $5 million in compensation were involved in a class-action IVC filter lawsuit in Florida that was transferred to the Southern District of Florida U.S. District Court in 2012. In 2015, all federal cases against C.R. Bard were ordered by the U.S. Judicial Panel on Multidistrict Litigation (MDL) to be centralized under MDL-2641 in the Southern District of Arizona.
A total of 3,834 class-action IVC filter lawsuits were pending against C.R. Bard as of May 2018.
MDL & Bellwether Trials against C.R. Bard
A bellwether trial sets precedent and serves as a reference point for a wider range of claims. After an MDL has been established, the judge and attorneys collaborate to choose bellwether cases. There is not a specified number of bellwether cases required, according to legal guidelines. The goal is to select a sampling of lawsuits that are representative of the whole. MDLs are non-binding, which means that they are not in any way restrictive of the proceedings in all other related cases.
The first bellwether trial for C.R. Bard was held in Phoenix, Arizona, beginning on March 30, 2018. Sherr-Una Booker was the plaintiff. A Bard G2 IVC filter had been implanted in 2007, and Booker suffered health complications after migration of the device and fracturing off of the device, which resulted in perforation of her inferior vena cava. To correct the damage, Booker underwent heart surgery. The federal jury in the case awarded a verdict of $3.6 million to the plaintiff. A total of $1.6 million in compensatory damages and $2 million in punitive damages was awarded to Booker.
In the second IVC filter lawsuit against C.R. Bard, the jury sided with the defendant on June 1, 2018. The plaintiff was Doris Jones of Georgia, who had a Bard Eclipse IVC filter implanted in 2010. The IVC filter fractured, and a piece blocked a major artery. Most of the fractured filter was removed, but doctors say that plaintiff is still at fatal risk because part of the device blocking the pulmonary artery could not be removed. The jury in the case ruled that Bard had provided doctors with adequate warnings regarding risks and potential complications.
IVC Filter Lawsuits against Cook Medical
As of May 2018, a total of 4,189 IVC filter lawsuits were filed against Cook Medical. A federal panel centralized the Cook Medical MDL in the southern district of Indiana. The first two IVC filter lawsuits against Cook were decided in favor of the defendant. In the first, Cook won in a complete jury verdict. In the second, the company won in a complete summary judgment verdict.
A Verdict of $1.2 Million Against Cook
The third verdict in an IVC filter lawsuit filed against Cook was announced on May 29, 2018. Plaintiff Jeff Pavlock, a firefighter, was awarded $1.2 million in a jury trial in Houston, Texas. The plaintiff’s claim was that he suffered organ and blood vessel perforations from IVC filter complications after a Cook Celect IVC filter was implanted in 2015 to prevent life-threatening pulmonary embolisms. The device was temporary. Once determined that the danger of a blood clot had passed, Pavlock’s doctors were planning to remove the medical device. Seven weeks after implanting the IVC filter, doctors found that they were unable to retrieve it because the medical device had migrated and become embedded in a blood vessel. A second surgery was done to remove the Cook Celect IVC filter, but it was also unsuccessful. Doctors say Pavlock requires lifetime health monitoring, as a result of the IVC filter complication. Cook announced that it is appealing the jury’s decision to award Pavlock $1.2 million.
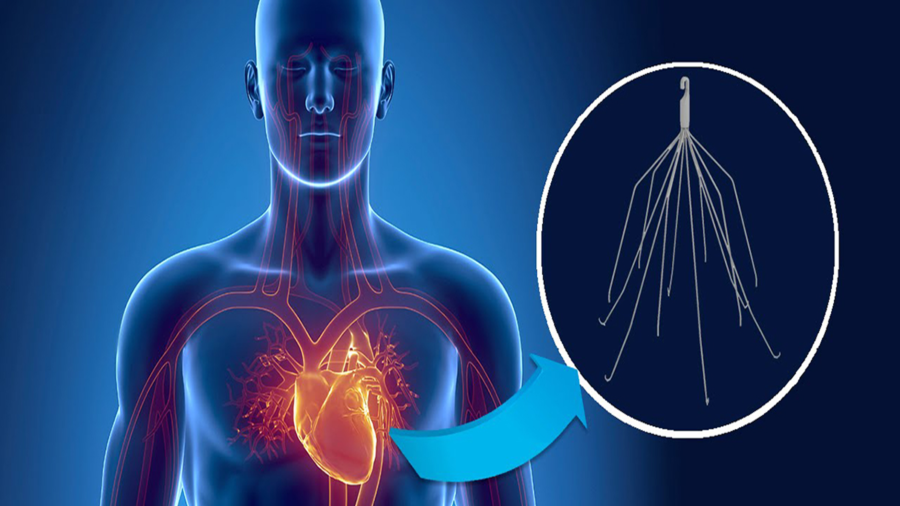
What is an IVC Filter?
A small spider-like medical device, an Inferior Vena Cava or IVC filter is designed for patients at risk of developing a life-threatening blood clot in the lung, known as a pulmonary embolism, or deep vein thrombosis (DVT). The device has been used when taking blood thinners is not a viable option for a patient.
IVC Filter Complications
IVC filters have been in use since 1979, when they were initially approved by the FDA. Originally, IVC filters were made with either stainless steel or titanium. Temporary IVC filters made with less expensive materials were later manufactured, as well. The FDA has issued various warnings through the years regarding the vena cava devices. For instance, from 2005 to 2010, adverse events reports released by the FDA included 202 incidents of filter fractures. Of the 202 events, 146 of them involved fractured pieces of vena cava medical devices migrating toward the heart.
FDA Recall of the Greenfield IVC Filter
Boston Scientific Corp. is the manufacturer of the Greenfield IVC Filter. Defects in the IVC filter have resulted in a growing number of federal lawsuits, but there is currently no multidistrict litigation docket (MDL) for the litigation. In one wrongful death lawsuit filed by the family of Cinthia K. Ratliff, the woman’s family alleged that she died in 2013 from severe injuries caused by the Greenfield IVC Filter. An autopsy confirmed their claim that her cause of death was inferior vena cava perforation caused by the IVC filter, resulting in retroperitoneal hemorrhage. A settlement agreement was reached in that case in 2017, and the case was dismissed.
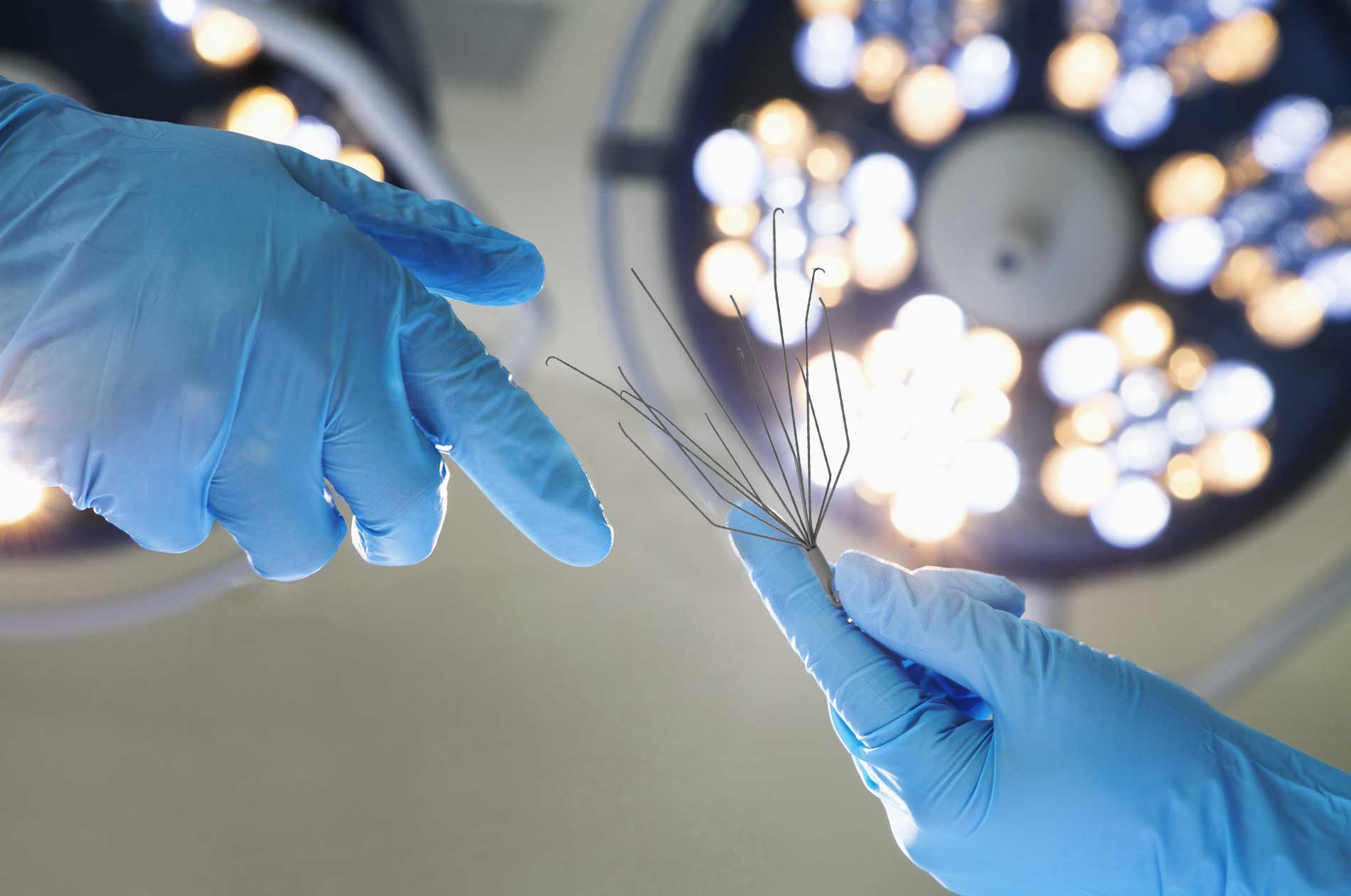
The basket-like Greenfield IVC Filter has six struts, each of which has a curved hook. Boston Scientific designed the medical device to catch blood clots. In May 2007, the Greenfield IVC Filter was recalled by the FDA following reports of a defect in which the bond between the carrier capsule and the filter’s outer sheath would detach, causing a risk of pulmonary and cardiac embolization.
FDA Recall of the Cordis OptEase IVC Filter
Under the controversial FDA 510(k) fast track approval process, companies manufacturing IVC filters have been allowed to engage in only minimal testing before placing the devices on the nationwide market for use in patients. Among the devices released through the fast track approval process were those manufactured by Cordis. In 2001, the FDA approved the Cordis TrapEase, which is a permanent IVC filter. In 2003, the Cordis OptEase, a retrievable vena cava filter, was released for use in patients.
Research studies were done which began to question the safety of IVC filters in general. The studies suggested that the medical devices are not only unreliable and create serious risks, but their effectiveness was called into question, in many cases. The Cordis filters, in particular, were found in studies to have a high failure rate, creating risks associated with filter fractures.
Half of all TrapEase IVC Filters implanted in patients became fractured after 50 months, on average, according to a 2012 study published in the prestigious JAMA Internal Medicine journal. After four years or more, the filters were fractured 64% of the time. Researchers concluded that there was an extremely high risk of strut fractures in the TrapEase IVC filter.
In 2013, Cordis informed medical professionals throughout the U.S. and Canada that there was a risk of serious injury or death in implanting the OptEase IVC filter because labeling problems could result in the filters being implanted backwards. The Cordis letter was classified by the FDA as a Class I medical device recall. This is the most serious FDA recall classification, indicating that the defects create the risk of serious adverse events, including possible death.
Have You Experienced IVC Filter Complications?
Do you suspect that you or a loved one who has had an IVC filter implantation is experiencing problems of some kind because of the medical device? The types of symptoms individuals experience as a result of IVC filter complications include:
- Nausea
- Confusion
- Neck pain
- Chest pain
- Fever
- Inflammation
- Hypotension
- Painful swelling in the legs
- Lower back pain
- Heart rhythm anomalies
- Shortness of breath
- Lightheadedness
- Internal bleeding or hemorrhaging
- Stroke
- Pulmonary embolism
- Loss of consciousness
- Death
When to File a Claim in an IVC Filter Lawsuit
If you or a loved one has experienced IVC filter complications, it is important that you speak with an experienced IVC filter lawyer as soon as possible. States each have their own statute of limitations for filing these kinds of claims. You may be entitled to seek compensation from the manufacturer and its subsidiaries for a wrongful death or the following past and future damages:
- Medical costs associated with the defective filter
- Lost wages
- Loss of capacity to earn money
- Loss of enjoyment of life
- Pain and suffering
You could be eligible to seek punitive damages, as well, if it is proven that the IVC manufacturer knowingly sold a defective filter. The types of lawsuits pending in state and federal courts against IVC manufacturers and their subsidiaries include claims of:
- Failure of the manufacturer of the IVC filter to provide adequate warnings regarding the potential dangers and side effects associated with implantation of the device;
- Defective designs of the vena cava IVC filter;
- Defective techniques in the manufacture of vena cava filters;
- Breach of implied warranty on the IVC filter; and
- Negligence on the part of the marketing company and/or manufacturer.
Each case involving complications is unique, and various factors determine the potential value of an IVC filter lawsuit. For example, what is the severity and overall magnitude of injuries suffered? How much time do doctors think it will take for the patient to heal from injuries suffered? Have the IVC filter complications resulted in a long-term or permanent disability?
Written by:

Stephanie McHugh
Stephanie McHugh is a professional writer who specializes in legal articles, technical blogs, and website copy. She is a former Official Court Reporter for a District Court in Houston, Harris County, Texas; newspaper columnist; and teacher. Stephanie is a professional writer with extensive experience reporting on health-related topics for publication. She developed an interest in health-related litigation as a court reporter while taking depositions for a class action lawsuit. Through her writing, she has been glad to help raise awareness about public health threats, to benefit victims.
Get a Free IVC Filter Case Review
If you or a loved one experienced a complication after getting an IVC filter, you may be eligible for money to pay for health care costs.